FDA Approves First 3D-Printed Mask for COVID-19 Support
Technological solutions could provide relief to medical facilities in urgent need of necessary equipment.
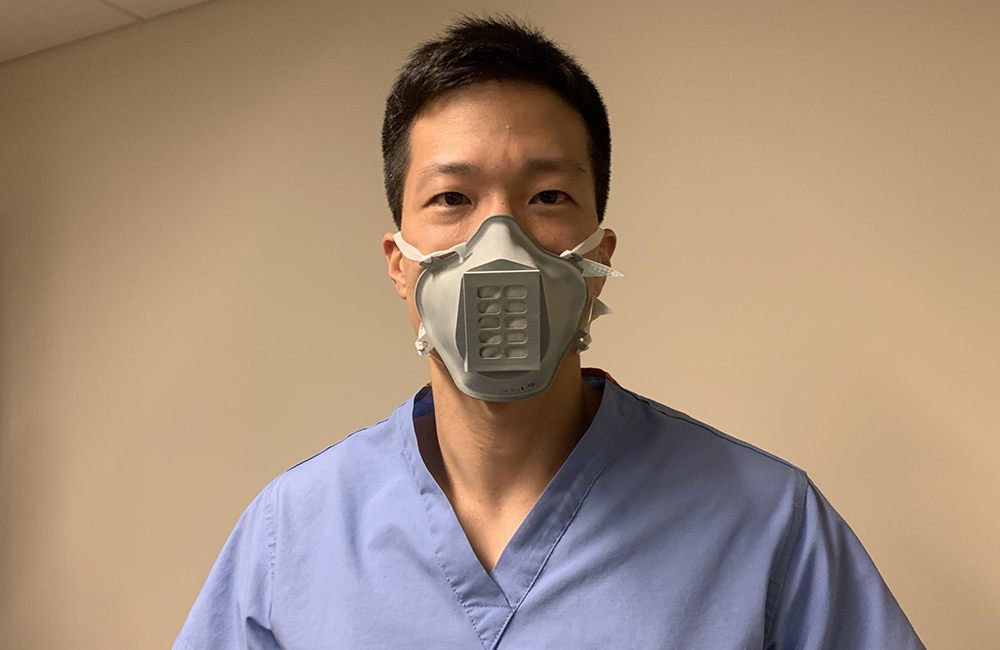
The Food and Drug Administration Friday approved the first 3D-printed mask to meet its regulatory standards to protect clinical health care professionals against COVID-19. The announcement comes from a tweet issued by the Veterans Health Administration Innovation Ecosystem.
Created by the VHA team, the 3D-printed Stopgap face mask is a personal protective mask health care workers can use for liquid barrier protection to the novel coronavirus.
The mask works by securely fitting around the mouth and nose, strapping to the ears. The mask is also easy to disinfect and includes a disposable filter piece for reuse.
According to the issued instructions for use, the mask should not be used as a replacement to traditional respirator masks approved as personal protective equipment (PPE), such as N95 masks, and is not suitable against airborne protection in high-level, inhalation-exposure clinical areas.
Respirators are the most protective masks against particulate transmissions, such as the viral aerosols spread by those infected with the virus.
The rapid approval of the 3D-printed mask comes amid reports of medical professionals lacking critical personal protective equipment while on the frontlines of the pandemic, in part due to a delay in global supply chains.
In response, the FDA had recently issued a memorandum of understanding between NIH, VHA and FDA. The public-private partnership is aimed at accelerating information-sharing and production of 3D-printed personal protective equipment and other medical device parts to quickly address the public health emergency.
The collaboration with additive manufacturer America Makes quickens the approval process for COVID-19 equipment using NIH’s 3D Print Exchange, an existing open-source tool that allows anyone who has a potential 3D-printing, biomedical prototype to upload and share them.
Those with COVID-19-related prototypes can submit them to the site, where they can then be clinically tested and evaluated by the VHA Innovation Ecosystem, in conjunction with the FDA via the VHA 3D Printing Network.
Once the models are tested, reviewed and approved, the final products are highlighted on the site. The medical equipment will also be matched and distributed to 3D-printing organizations that can produce them effectively and distribute them to health care facilities in need.
So far, there have been seven COVID-19-related, 3D-printed medical devices that have been “reviewed for clinical use,” with the mask approved a week after the effort’s launch March 27.
This is a carousel with manually rotating slides. Use Next and Previous buttons to navigate or jump to a slide with the slide dots
-

How Tech Enables Environmental Justice at EPA
The agency wants to eliminate bias and establish new tech standards to reduce greenhouse gas emissions.
39m listen -

The CAIOs Leading Responsible AI Development Across Government
Since the White House's AI executive order, federal agencies are in the process of naming chief artificial intelligence officers.
7m read -

How TMF is Helping Agencies Accelerate Tech Modernization
The program launched a new AI pilot to expedite TMF applications as agency leaders urge more to consider applying for funds.
4m read -

Defense Board to Pitch Solutions for Closing Tech Talent Gaps
Defense Innovation Board members cite need to modernize people management the same way government modernizes technology.
4m read



